This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Catalyst search shows how computing can take the guesswork out of chemistry
Imagine synthesizing and then testing over 50 different complex molecules to identify the most effective catalyst for a particular chemical reaction. The traditional approach to developing new catalysts for chemical reactions in this "try it and see" manner is often extremely labor intensive, requiring numerous repeated experiments with potential candidate molecules. The now ubiquitous technique of machine learning can make this task much more efficient by predicting the performance of catalysts ahead of time based on theoretical characteristics.
In a study published in Nature Communications, researchers from Osaka University used a computer library of molecules that have been synthesized together with molecules that are entirely theoretical at the moment to find the best catalyst for a specific chemical reaction.
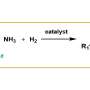
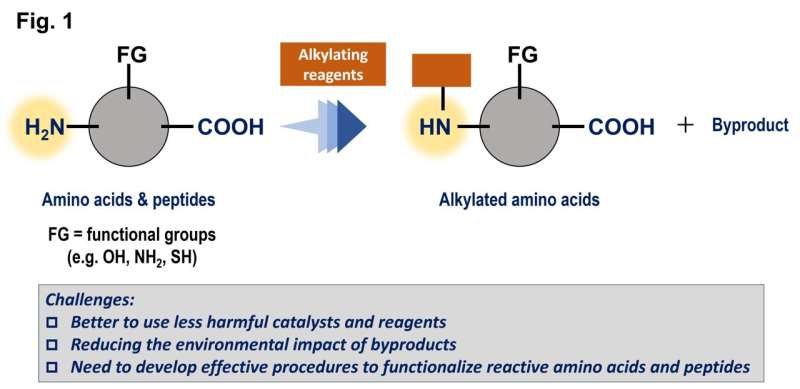
The objective of the work was to find better ways to add groups of carbon to amino acids and peptides, which are very common in living organisms, to modify the properties of these compounds. Like many reactions, these processes are enhanced by catalysts, but a traditional metal-based catalyst is often toxic and/or expensive.
This study aimed to use triarylboranes as catalysts, but because of their relatively complex structures, there are potentially hundreds of possibilities. These compounds are based on boron, which is a main group element that is relatively inexpensive and less toxic.
"The assessment of molecular catalysts for organic synthesis can be extremely time-consuming," says lead author of the study Yusei Hisata. "In the case of the triarylboranes used in our work, many permutations of molecular structures could require months of study just to identify the optimum candidate."
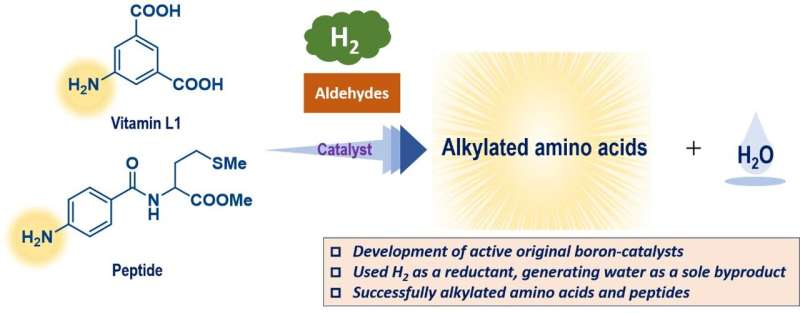
The researchers combined experimental data from a limited number of synthesized triarylboranes with properties predicted for other molecules that have not yet been synthesized, using theoretical calculations, to make a library of 54 possible catalysts.
"This process assessed parameters that we predicted would affect the reaction progress," explains Yoichi Hoshimoto, the corresponding author. "These included factors such as the molecular orbital energy levels and the energy barriers to certain processes."
A Gaussian process regression using the in silico library identified a promising candidate, and tests with this triarylborane demonstrated a high level of performance. This compound could promote the reactions of an amino acid in very high yields and tolerate the presence of numerous different functional groups. As an added benefit, these reactions generate only water as a harmless coproduct because they successfully used molecular hydrogen, H2, as a reagent.
This work also examined other ways to lower the environmental impact of the process and found that the hazardous solvent tetrahydrofuran could be replaced with the less toxic alternative 4-methyltetrahydropyran.
Modern-day chemists face mounting demands, and they juggle developing new syntheses with limited peers while considering environmental impact, efficiency, cost, sustainability, and other factors. This study demonstrates an important step forward in the use of machine learning to streamline the development of new chemical processes and highlights how these new processes can incorporate changes that work together to generate green systems.
More information: Yusei Hisata et al, In-silico-assisted derivatization of triarylboranes for the catalytic reductive functionalization of aniline-derived amino acids and peptides with H2, Nature Communications (2024). DOI: 10.1038/s41467-024-47984-0
Journal information: Nature Communications
Provided by Osaka University